Industrial Design, Factory Design, Laboratory Buildings, Large Scale Architecture
RioCare Pharmaceutical Plant
“Production units and factories can be elegant and distinct without functional compromise and significant cost escalation.” - the client and Studio Code embraced this concept early in the design process.
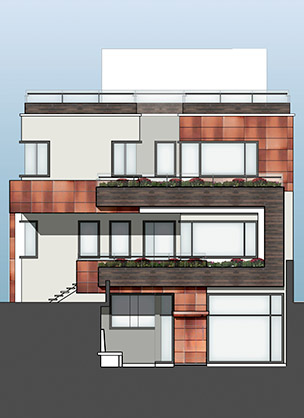
The design attempts to create a distinct visual characteristic within the complex functional requirements of a pharmaceutical plant. Significant potential expansion has been taken into account. A multi-functional, expandable, wrapping ribbon was envisaged for the façade.
The project received an US-FDA (Food and Drug Administration) and WHO-GMP (Good Manufacturing Practice) accreditation, as the finishes, materials, architectural design and details conform to international standards for pharmaceutical facilities. We are proud to be partners with RioCare India Pvt. Ltd. as this certification signifies competency in the design and functionality of the facility.
Studio Code followed a two-pronged design approach of analyzing and integrating the plant requirements and simultaneously conducting aesthetic studies. Flow diagrams were created for men and materials while aesthetic massing and functional massing models were studied.
The ground floor was designed as the primary production floor. Two cores were created – one for the office section and the second for services. The two-core system also allowed the building to expand vertically in two separate sections based on the clients’ possible future requirements of production, storage or office space. An internal courtyard was designed on the first floor as an anchoring element for all the staff and office areas. The final design visually demonstrates the tier separation of the production area and offices.
An aesthetic concept of a ‘ribbon’ wrapping around the building was selected. The ribbon starts as two strips on the front and changes size as it clads the various sides of the building, creating the visual illusion of masking the number floors. A metal louvered cladding was selected for the ribbon to hide functional openings and ventilators. Additionally, the ribbon concept allows for vertical expansion as the cladding area can also expand to clad the new building surfaces.
-
SIZE:
30,000 SQ.FT. / 2,800 SQM
-
STATUS:
COMPLETED
-
DESIGN PRINCIPAL:
VIKAS KANOJIA, RAJIV BHAKAT
-
DESIGN TEAM:
PALLAVI RANI, SHUBH CHEEMA, DIGHAMBER BISHT, DEEPIKA TIWARI, VIDHU SAXENA, MEGHA VERMA, MITALI KEDIA
-
COLLABORATORS:
CHORDIA TECHNO CONSULTANTS - STRUCTURE, SPECTRUM PHARMA CONSULTANT - PHARMACEUTICAL+ELECTRICAL+PLUMBING+HVAC, I.M. ASSOCIATES - TENDER+BOQ, BIOASSET TECHNOLOGIES PVT. LTD. - LAB CONSULTANT, CIVIL-EXPERT CONSTRUCTION, KELVOLT-MEP